Brexit & Beyond newsletter
14 January 2025
Welcome to the 14 January 2025 Brexit & Beyond newsletter
Happy New Year from all of us at Brexit & Beyond.
Since the last issue, the Windsor Framework Democratic Scrutiny Committee has met twice. It has published two reports and is currently seeking views on two other pieces of EU legislation.
The Minister for Communities wrote in December that his Department and Sport NI are still assessing the impact of the EU chemical REACH legislation on Northern Ireland including potential impacts on synthetic surfaces such as 3G pitches. The European Commission has confirmed a transition period before new restrictions on microplastics becomes effective in October 2031.
Last week, the Secretary of State for Northern Ireland, announced that Lord Murphy of Torfaen will conduct the independent review into the functioning of the Windsor Framework.
From 1 January 2025, the UK’s Medicines and Healthcare products Regulatory Agency has full responsibility for regulating medicines in Northern Ireland as a result of the Windsor Framework.
From Wednesday 8 January 2025 all eligible non-Europeans visiting or transiting through the UK without a visa will need to obtain an electronic travel authorisation.
- Windsor Framework Democratic Scrutiny Committee
- EU chemical REACH legislation
- Independent Review into the functioning of the Windsor Framework
- Multilateral Free Trade
- Electronic Travel Authorisation Scheme
- Regulating medicines in Northern Ireland
- The British Chambers of Commerce: The Trade and Cooperation Agreement Four Years On
- Other news
Windsor Framework Democratic Scrutiny Committee
On 19 December, the Windsor Framework Democratic Scrutiny Committee agreed two reports for inquiries on Directive (EU) 2024/2853 on liability for defective products and Regulation (EU) 2024/2865 on classification, labelling and packaging of substances and mixtures.
Directive (EU) 2024/2853 repeals and replaces the existing Product Liability Directive 85/374/EEC (PLD), updating the rules around 'strict liability', where consumers can claim compensation for defective products without having to prove fault on the part of the manufacturer. It aims to enhance consumers' access to compensation, for example by expanding the scope of liability rules to encompass software products (including Artificial Intelligence) and electronic goods that operate using software, such as autonomous vehicles. It also aims to ensure consumers have a liable party to pursue if damage or injury is caused by a faulty good they bought online from outside the EU Internal Market.
In reaching its conclusions, the Committee considered all the evidence provided to it. It focused on the two conditions that must be satisfied if the Stormont Brake is to be pulled. Though the Committee determined that the replacement EU act significantly differs, in whole, from the content or scope of the EU instrument it replaces, it resolved that the replacement EU act would not have a significant impact specific to everyday life of communities in Northern Ireland in a way that is liable to persist.
Regulation (EU) 2024/2865 on classification, labelling and packaging of substances and mixtures amends Regulation 1272/2008 on classification, labelling and packaging of substances and mixtures (the 'CLP Regulation'). The CLP Regulation implements the UN globally harmonised system (GHS) for classification and labelling of chemicals. It aims to protect human health and the environment while ensuring a level playing field for all suppliers of chemical substances and mixtures in the EU single market.
The amendments to the CLP Regulation are intended to:
- improve protection against hazardous chemicals by ensuring they are identified and classified appropriately and in the same way across the EU;
- improve communication of hazards by making labels more accessible and understandable for users of chemicals (e.g. minimum font size, greater use of fold-out labels, voluntary digital labelling of chemicals);
- provide companies with more flexibility;
- address legal gaps and ambiguities of CLP provisions and strengthen compliance (chemicals suppliers must be established in the EU (or NI), including for distance sales); and
- adapt CLP requirements to apply to different methods of sale e.g. refillable containers and online sales.
In reaching its conclusions, the Committee considered all the evidence provided to it. It focused on the two conditions that must be satisfied if the Stormont Brake is to be pulled. Having considered its commissioned legal advice, the Committee concluded that the replacement EU act significantly differs, in part, from the content or scope of the EU instrument which it amends.
Having considered evidence received from the UK Government, the Department for the Economy, the Department of Health, the Department of Justice, and the responses to its Citizen Space survey, the Committee concluded that it was unable to reach a view on whether the replacement EU act would have a significant impact specific to everyday life of communities in Northern Ireland in a way that is liable to persist.
Read more information on the Stormont Brake here.
Last Thursday, the Windsor Framework Democratic Scrutiny Committee considered legal advice and departmental evidence in relation to three EU Acts:
- Regulation (EU) 2024/3110 laying down harmonised rules for the marketing of construction products and repealing Regulation (EU) No 305/2011
- Regulation (EU) 2024/3115 amending Regulation (EU) 2016/2031 and Regulation (EU) 2017/625 regarding plant health controls and reporting obligations
- Regulation (EU) 2024/3234 amending Regulation (EU) 2023/1115 as regards provisions relating to the date of application of the EU Deforestation Regulation
Regulation (EU) 2024/3110 laying down harmonised rules for the marketing of construction products and repealing Regulation (EU) No 305/2011 is part of a package of European Green Deal proposals intended “to make almost all physical goods on the EU market more friendly to the environment, consistent with circular business models, and energy efficient throughout their whole lifecycle from the design phase through to daily use, repurposing and end-of-life.”
The Committee decided to conduct an inquiry into this EU act and is holding an online survey and wants to hear from those who may be affected by the EU act. You can take part in the survey which closes on 19 January here.
Regulation (EU) 2024/3115 amending Regulation (EU) 2016/2031 and Regulation (EU) 2017/625 regarding plant health controls and reporting obligations. The EU Plant Health Regulation is one of the principal regulations of the plant health regime and establishes lists of regulated pests and products in line with prescribed criteria based on phytosanitary principles. The amendments to the EU Plant Health Regulation by COM(23)661 are proposed to enhance its effectiveness, transparency, and the practical implementation of EU rules for plant health. The Commission emphasise that these amendments do not change the EU’s plant health policy but aim to improve the application of the existing policy.
The Committee decided to conduct an inquiry into this EU act.
The Committee is holding an online survey and wants to hear from those who may be affected by the EU act. It closes on 19 January.
The Committee decided not to conduct an inquiry into Regulation (EU) 2024/3234 regarding the date of application of the EU Deforestation Regulation.
The Committee also noted COM/2024/577 Proposal for a Regulation amending Regulations (EU) No 1308/2013, (EU) 2021/2115 and (EU) 2021/2116 as regards the strengthening of the position of farmers in the food supply chain which it was notified of on 2 January. The Committee agreed to seek legal advice and an initial departmental assessment of the impact of the proposal.
EU chemical REACH legislation
In response to Assembly Written Question 17195/22-27 the Minister for Communities wrote in December that his Department and Sport NI are still assessing the impact of the EU chemical REACH legislation on Northern Ireland. The Minister set out that this “legislation will ban the sale of intentionally added microplastics into the European market, including rubber infill for synthetic surfaces such as the 3G pitches which have been installed across Northern Ireland. The European Commission has confirmed a transition period before the new restriction becomes effective in October 2031.”

The Minister confirmed“there is no immediate requirement to remove existing rubber crumb infill from artificial grass pitches, however it is expected that it will not be possible to source new rubber crumb to maintain existing artificial grass pitches from October 2031.”
Regarding the financial impact of this legislation on the sector, the Minister wrote that it is normal practice that for any 3G pitch funded by the Department or Sport NI, the asset owner will consider the lifecycle and maintenance costs for the project as part of its initial Business Case assessment stage.
Independent Review into the functioning of the Windsor Framework
In December, the Assembly voted on the continued application of Articles 5 -10 of the Windsor Framework. The motion passed with a simple majority of the elected members voting in favour (without cross-community support).
As set out in Schedule 6A of the Northern Ireland Act 1998, the Secretary of State for Northern Ireland is obliged to commission an Independent Review into the functioning of the Windsor Framework, in accordance with paragraphs 7 - 9 of the Unilateral Declaration of October 2019.
On Thursday 9 January, the Secretary of State for Northern Ireland, Hilary Benn MP made a written statement announcing that Lord Murphy of Torfaen will conduct this Review.
Lord Murphy of Torfaen
Lord Murphy previously served in Government as Minister of State for Northern Ireland, Secretary of State for Northern Ireland and Secretary of State for Wales.
In line with the Review’s terms of reference, published on Friday, Lord Murphy must provide the Secretary of State with a report of the review’s conclusions no later than six months after having been commissioned.
Multilateral Free Trade
Last Monday in the House of Lords, Lord Leong (Government Whip), reiterated the Government’s commitment to “multilateralism and a rules-based trading system with the WTO at its core.” He also said the “Government will continue to make a very strong case for free trade.”
Regarding the UK and the Comprehensive and Progressive Agreement for Trans-Pacific Partnership (CPTPP) and Lord Leong said:
“The UK is the first country to accede to the CPTTP and the first European member of the CPTPP, demonstrating the Government’s commitment to multilateral trade.”
The CPTPP is an agreement between a number of trans-Pacific countries including Australia, Canada, Japan, Mexico, New Zealand and Singapore.
Electronic Travel Authorisation Scheme
From Wednesday 8 January 2025 all eligible non-Europeans visiting or transiting through the UK without a visa will need to obtain an electronic travel authorisation (ETA).
An ETA is an advance digital permission to travel – it is not a visa and does not permit entry into the UK – it authorises an individual to travel to the UK.
An ETA costs £10 and permits multiple journeys to the UK for stays of up to six months at a time over two years or until the holder’s passport expires – whichever is sooner.
All European visitors will require an ETA from 2 April 2025. British and Irish citizens are exempt.
The Economy Minister, Conor Murphy MLA, has urged the Home Office to reconsider their implementation of the new scheme which he believes will have a devastating impact on tourism in Northern Ireland. Speaking on 7 January, he said the UK Government should adopt a "pragmatic and flexible approach" and put in place an exemption for tourists who were crossing the land border from the Republic of Ireland into Northern Ireland but not travelling on to Great Britain.
The Department for the Economy also pointed to findings from recent Tourism Ireland Sentiment Research across international markets that found that due to the introduction of the ETA requirements 1 in 4 people in Europe, and 1 in 5 people in North America say they would make the decision not to travel.
Regulating medicines in Northern Ireland
From 1 January 2025, the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) has full responsibility for regulating medicines in Northern Ireland as a result of the Windsor Framework.
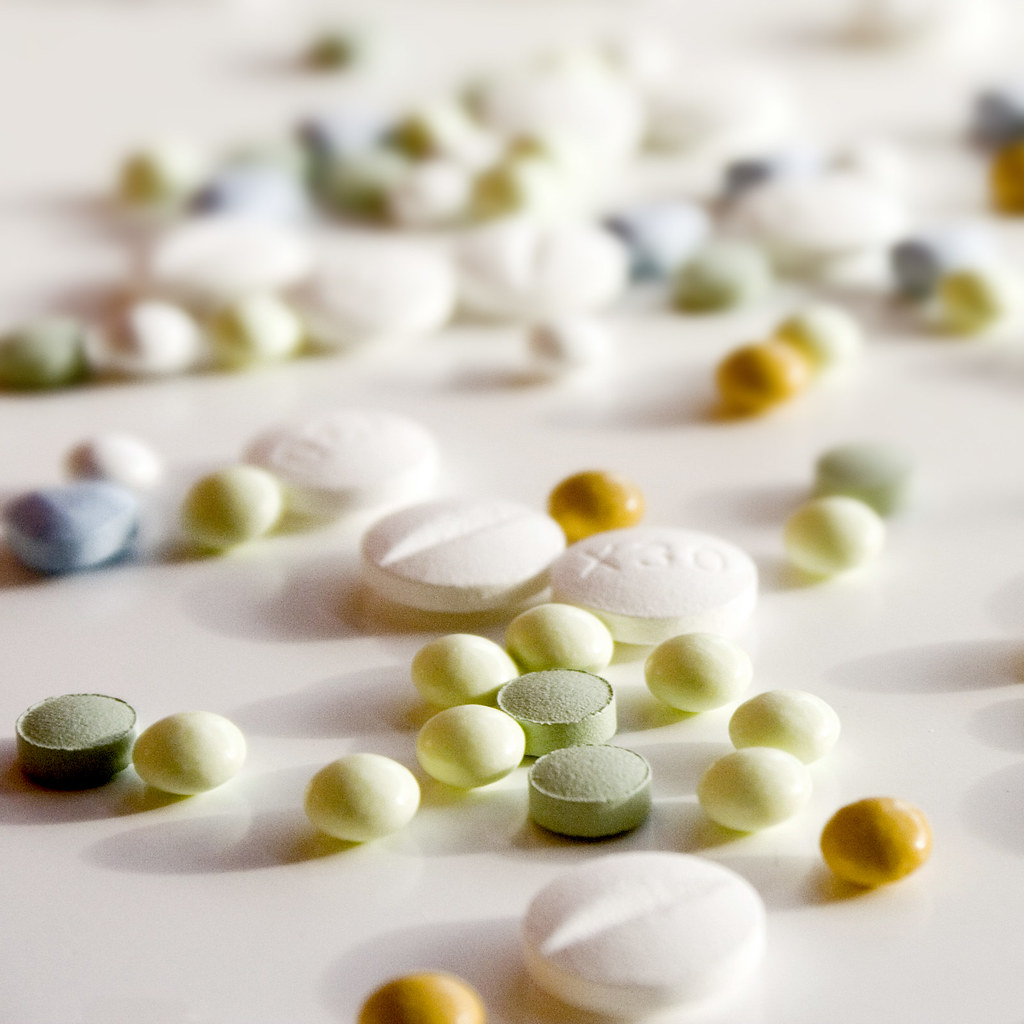
Regulating medicines in Northern Ireland is now the responsibility of the UK’s Medicines and Healthcare products Regulatory Agency
These products will only be able to be sold in the UK and export markets where it is lawful for them to be placed on the market or supplied as unlicensed medicines in those markets. These products cannot be supplied to the EU, including the Republic of Ireland, unless in compliance with regulatory pathways and guidance from respective national competent authorities.
The major practical change as a result of the new deal is that packaging for all relevant products on the UK market must now carry a "UK only" label.
The EU Falsified Medicines Directive (EU FMD) safety features will no longer apply in NI from 1 January 2025.
The British Chambers of Commerce: The Trade and Cooperation Agreement Four Years On
At the end of December, the British Chambers of Commerce (BCC) published its report of its annual International Trade Survey which ran from July to August 2024. This received 1,111 responses from businesses (92% SMEs) across the UK. Additionally, 45% of respondents reported that they export.
BCC said the results “shows the urgency for the Government to reset trade relations with the EU is increasing.” The report sets out priorities for any reset negotiations in 2025 taking place after the Leaders’ Summit in the spring. These include:
- Negotiate a deep veterinary or animal origin and plant product (SPS) agreement with the EU. This must either reduce the complexity of, or preferably eliminate, the need for Export Health Certificates and associated checks on agri-food imports and exports between GB and the EU.
- Agree a critical imports and supply chains accord between the UK and the EU.
- Produce a comprehensive Youth Mobility scheme between the UK and EU, covering school visits and exchanges, and the ability to work for young people, under time-limited visas.
Other news
- The Guardian reports that Miguel Berger, Germany’s ambassador to the UK, has said that the youth mobility and the ERASMUS schemes are important elements of the reset in UK-EU relations.
- It was announced on 8 January that Sir Oliver (Olly) Robbins has been appointed as the new Permanent Under-Secretary at the Foreign, Commonwealth & Development Office. He was formerly Permanent Secretary at the Department for Exiting the European Union before becoming chief Brexit negotiator under then-prime minister Theresa May. He left the UK Government in 2019. The Cabinet Secretary also announced today that Michael Ellam has been appointed as Second Permanent Secretary, European Union and International Economic Affairs in the Cabinet Office. The Cabinet Office states “this is a new role, leading official-level discussions with the EU and in international forums such as the G7 and G20 to support the UK’s economic growth and national security – the foundations of the Government’s Plan For Change. Michael will also manage the EU Relations Secretariat in the Cabinet Office, set up by the Prime Minister in July to deliver the UK’s resetting of relationships with the EU and secure closer links in areas like trade and security.”
- Last Monday (6 January), the Independent reported that Daniel Zeichner MP, Minister of State at the Department for Environment, Food and Rural Affairs, had said that British food sales to Europe have fallen by a fifth since 2018.





