Brexit & Beyond newsletter
3 June 2024
 Welcome to the 3 June 2024 Brexit & Beyond newsletter
Welcome to the 3 June 2024 Brexit & Beyond newsletter
The Windsor Framework Democratic Scrutiny Committee has published its first report. The Committee for Health was briefed by departmental officials on the Windsor Framework and supply and regulation of medicines and medical devices for Northern Ireland. Elections to the European Parliament take place on 6-9 June.
- Windsor Framework Democratic Scrutiny Committee
- Committee for Health: Medicines and the Windsor Framework
- European Parliament elections
- Other news
Windsor Framework Democratic Scrutiny Committee
The Windsor Framework Democratic Scrutiny Committee has published its first report. It sets out the conclusions of its inquiry into a published replacement EU act, Regulation (EU) 2024/1143 on geographical indications for wine, spirit drinks and agricultural products.
In reaching its conclusions, the Committee focused on the two conditions to be satisfied for the Stormont Brake to be used:
- the content or scope of the replacement EU act “significantly differs”, in whole or in part, from that which it replaces
- the application in Northern Ireland of the replacement EU act, or of the relevant part, “would have a significant impact specific to everyday life of communities in Northern Ireland in a way that is liable to persist”
These terms are set out in Article 13(3a) of the Windsor Framework. The Windsor Framework (Democratic Scrutiny) Regulations 2024 state that the Democratic Scrutiny Committee must have regard to this when deciding whether to conduct conducting an inquiry. Read more about the Stormont Brake and the work of the Committee on our website.
Having considered its legal advice, the Committee concluded that the replacement EU act “significantly differs, in part, from the content or scope of the EU instrument which it amends or replaces.”
The Committee considered the UK Government’s impact assessment, evidence from the Department of Agriculture, Environment and Rural Affairs, and responses to its Citizen Space survey. It took the view that “for an act to have a significant impact…that significant impact must be negative.” The Committee concluded that the replacement EU act “would not have a significant impact specific to everyday life of communities in Northern Ireland in a way that is liable to persist”.
Committee for Health: Medicines and the Windsor Framework
On 30 May, the Committee for Health was briefed by departmental officials on Brexit issues, including the Windsor Framework, and its impact on supply of medicines and medical devices to NI.
Previously under the Protocol, EU pharmaceutical law applied in Northern Ireland, which caused serious concern about the future supply of medicines from Great Britain to Northern Ireland. Chief Pharmaceutical Officer Cathy Harrison told the Committee that while the EU made legislative changes in April 2022 to ensure the continued long-term supply of medicines from GB to NI, issues remained. NI was required to comply with the EU Falsified Medicines Directive (FMD) for medicines packaging; and all novel and innovative medicines under the scope of the EU's centralized authorisation procedure (CAP) had to be authorised by the European Commission for Northern Ireland.

Chief Pharmaceutical Officer Cathy Harrison
Harrison explained the mitigations which were put in place: the NI MHRA Authorised Route (NIMAR) permits the supply of GB authorised medicines to NI to meet patients’ unmet needs. There are 280 medicines on the NIMAR list as of 28 May. Additionally, in April 2022 the EU centralized authorised procedure bridging mechanism was introduced. This means the UK regulator, the MHRA, can temporarily authorise medicines for NI for six months or until a decision is made by the EU. This system has only been used twice. Harrison said these mitigations have been “really important for us and we rely on them currently, and they've been very useful in ensuring patients access to medicines.”
However, Harrison emphasised that they needed “long-term sustainable solutions” for NI’s medicine supply chain. She said the Windsor Framework addressed some of the outstanding regulatory concerns and “does present a more long-term solution to Northern Ireland's medicine supply chain.” The Windsor Framework disapplies the FMD packaging requirements; removes NI from the EU centralized authorisation procedure for novel and innovative medicines marketed in the UK; and makes the MHRA NI’s only regulator of medicines. Medicines must be labelled ‘UK only’. The changes are to be implemented from 1 January 2025 and “work is well under way” on implementation and legislation. The UK Government is expected to bring forward legislation in July to give effect to the changes.
Harrison told the Committee that regarding medicines, “We're hoping that the issues will be resolved with Windsor Framework.” She said the issue of divergence in timelines between the UK and EU regulators’ approval of medicines will stop, as NI will have only one medicines regulator, the MHRA. They may need to continue to make use of the NIMAR system, which she called “a great backstop”.
Medical devices
Harrison noted that supply of medical devices and in vitro diagnostics were not addressed by the Windsor Framework. Relevant EU regulations in this area apply. She told the committee that as GB and Northern Ireland are following different regulatory requirements, divergence “can have an impact on supply of medical devices at times”. The Department works with the UK Department for Health and Social Care on these matters and monitors supply lines. Although there have been a few discontinuations, there have been alternatives available, so this issue hasn’t impacted the health service.
Regulatory divergence
The Committee heard that the Department for Health “has taken steps to manage this new and emerging area of risk, and to ensure, where possible, early identification of potential divergence.” Its policy officials are required to ‘horizon scan’ for potential divergence. Harrison noted some potential areas of divergence: EU proposals to ban dental amalgam (which has been considered by the Democratic Scrutiny Committee), rules on substances of human origin, and proposed EU pharmaceutical reforms. Harrison stated, “Identifying these issues at an early stage is absolutely critical so that we can understand the implications and what, if any, action need to be taken”. She pointed to the governance structures in the Windsor Framework for the UK Government and the EU to discuss any concerns and risks.
Monitoring and surveillance
An official explained to the Committee that the Windsor Framework has heightened the requirements and responsibilities for monitoring and surveillance of goods such as medicines, controlled drugs, poisons, and some chemicals in Northern Ireland. He said the European Commission recognises that that there are some areas of potential EU-UK divergence and “requires assurances that there's adequate monitoring” of such goods to ensure those which may not be compliant with EU standards do not enter the EU single market. He explained that new labelling requirements would allow for early identification if they enter the EU single market. The UK Government must provide the European Commission with a written guarantee that there is adequate monitoring and surveillance of these products.
European Parliament elections
Elections to the European Parliament take place on 6-9 June. The European Council on Foreign Relations (ECFR) predicts a “sharp turn to the right”. The Centre for European Reform also makes this assessment and notes, “parties within the current ‘grand coalition’ of conservatives, socialists and liberals are likely to lose a substantial number of seats but maintain their overall majority”. Politico’s poll of polls projects the likely results of the election.
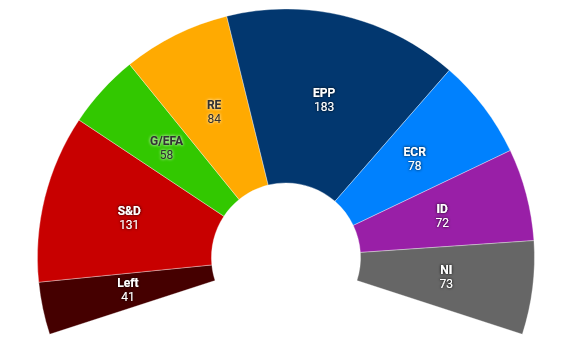
Projected make-up of the European Parliament | Source: Simon Hix and Kevin Cunningham
At the European Council on 27-28 June, the top jobs - Commission President, Council President, High Representative for Foreign Affairs and Security Policy, and President of the European Central Bank - are expected to be agreed by member states. The European Parliament has published an article on the next steps following the election: at the first plenary session starting on 16 July, the new Members of the European Parliament (MEPs) will elect their President. Parliament will then decide on legislative proposals which were not concluded before the election, which include combatting corruption, AI liability, animal transport and the welfare of cats and dogs.
Later this year, MEPs will elect the new European Commission President, nominated by leaders of EU countries. The newly appointed Commissioners will face hearings before parliamentary committees before being approved in a plenary vote. This vote is expected to take place in the autumn.
The next meeting of the European Political Community, which brings together around 50 leaders from across Europe, is being hosted by the UK on 18 July.
Other news
- The fourth UK-EU annual report on the functioning of the Withdrawal Agreement for 2023 has been published. It provides an overview of UK-EU activity and meetings of the committees in the governance structure of the Withdrawal Agreement. It concludes that meetings of these committees “continued to provide a stable basis for dialogue and cooperation by both parties to facilitate the implementation and application of the Withdrawal Agreement and to seek solutions to outstanding issues”. Read more about the governance of the Withdrawal Agreement.
- The Specialised Committee on Citizens Rights is meeting on 6 June. The Committee will hear from civil society organisations on issues encountered by EU citizens in the UK and UK nationals in the EU. It will also discuss new EU and UK border schemes: the EES (Entry/Exit System), ETIAS (European Travel Information and Authorisation System) and ETA (Electronic Travel Authorisation).
- The European Parliament think tank has published an infographic on the economic performance of the UK and EU, which also considers EU-UK trade dynamics.
- The Scottish Parliament Constitution, Europe, External Affairs and Culture Committee has published its annual report. It covers the Committee’s work on matters including devolution after Brexit, inter-parliamentary engagement, and its inquiry into the Review of the EU-UK Trade and Cooperation Agreement.
- Labour leader Sir Keir Starmer has said there is “huge scope” for closer work with the EU on defence, security and education.



