Brexit & Beyond Newsletter
20 December 2021

Welcome to the 20 December 2021 Brexit & Beyond newsletter
Lord Frost has resigned as Brexit Minister. The EU is advancing its proposals on medicines supply to Northern Ireland. The Committee for the Executive Office considered evidence on Article 2 and rights of individuals in the context of Brexit. The Committee for Health heard evidence on medicines supply in relation to the Protocol, and on Common Frameworks. The UK will not yet impose full border controls on the Republic of Ireland. We wish our readers a Merry Christmas and Happy New Year!
Lord Frost’s resignation
Lord Frost has resigned from his position as UK Brexit Minister. In his letter to the Prime Minister, he says that the process of building a new relationship with the EU is “a long-term task”. He says the challenge for the UK Government is to deliver on the opportunities of Brexit and refers to his “concerns about the current direction of travel”. UK Foreign Minister Liz Truss has taken over responsibility for relations with the EU. Chris Heaton-Harris is now the Minister of State for Europe.

Lord Frost, who has stepped down as UK Brexit Minister | Source: UK Parliament
Movement on medicines
The EU is moving ahead with its proposals on medicines. Lord Frost and European Commissioner Vice President Maroš Šefčovič met twice last week. Frost released a statement following their Friday meeting: he said his preference was to reach a solution “dealing with all the issues” but they have been “prepared to consider an interim agreement as a first step to deal with the most acute problems.” He said it was “disappointing” that this was not possible this year. Lord Frost said of the Commission’s proposals, “they do not solve the problems, and even in some aspects take us back from the current unsatisfactory status quo.” In his statement, Vice-President Maroš Šefčovič said, “Ensuring prosperity and stability on the island of Ireland…has been at the heart of the Commission's tireless engagement.”
Medicines
The EU has moved to deliver on its proposal on medicines, which it says will mean everyone in Northern Ireland will have access to the same medicines at the same time as elsewhere in the UK. It will also ensure continued supply of medicines to Cyprus, Ireland and Malta, EU countries which have historically been dependent on medicines from the UK. Lord Frost said this was the main area of progress and that the EU’s proposals “could constitute a constructive way forward, and we are willing to look at them positively, but as we have not been able to scrutinise the texts in the necessary detail we are not yet able to make that judgement with full confidence.”
The EU will change its own legislation to ensure the continued and timely supply of medicines to NI. The Commission’s press release contains more details of the proposal, which will allow generic medicines to be authorised under UK procedures, will have a ‘bridging solution’ to allow innovative medicines authorised in the UK to be supplied in NI at the same time as the rest of the UK. The Commission says the UK will need to respect packaging requirements to ensure that UK-authorised medicines don’t enter the EU Single Market, and that the UK assuming sole responsibility for authorising medicines for Northern Ireland is “contingent on the UK complying substantively with EU law on quality, safety and efficacy of human medicines when issuing market authorisations for Northern Ireland”.
The Association of the British Pharmaceutical Industry and European Federation of Pharmaceutical Industries and Associations (EFPIA) welcomed the “pragmatism” shown by the EU and UK and said, "These developments, and the constructive dialogue of the past few weeks, puts us on a strong footing for a long-term resolution." Until the new regime is in place, the existing ‘standstill’ period will continue until the end of 2022 unless the legislative change is finalised sooner. The Commission’s proposals will now be sent to the European Parliament and Council. The Commission has also published a Q&A on the proposals. Discussions on veterinary medicines will continue.
Democratic deficit
Šefčovič emphasised that “strengthening engagement with authorities and stakeholders in Northern Ireland to ensure that their voices are better heard in the implementation of the Protocol remains high on our agenda.” He regrets that the UK Government has not engaged with its proposal to give stakeholders a greater role. In the House of Lords on Thursday, Lord Frost was asked about addressing the democratic deficit and a decision-making role for MLAs in relation to EU legislation. He said, “it would not be a good solution to give the Northern Ireland Assembly or Executive decision-making roles in the European Union. The UK is not a member of the European Union, and therefore it would not be right or appropriate to try to resolve these questions in that way”.
The House of Lords Sub-Committee on the Protocol wrote to Lord Frost on the democratic deficit last week. The Committee regrets the lack of specific proposals in the UK’s Command Paper, recommending it bring forward proposals to address this “on the basis of full consultation with the Northern Ireland Executive, together with representatives of all communities in Northern Ireland, civic society and other stakeholders.” The Committee welcomes the EU’s proposals on engagement, especially on transparency in the governance structures, however it says they are insufficient to remove the democratic deficit under the Protocol. The Committee recognises that more formalised mechanisms for NI’s engagement with the EU institutions would be “politically contentious” and seeks the Government’s views on various proposals put forward by stakeholders.
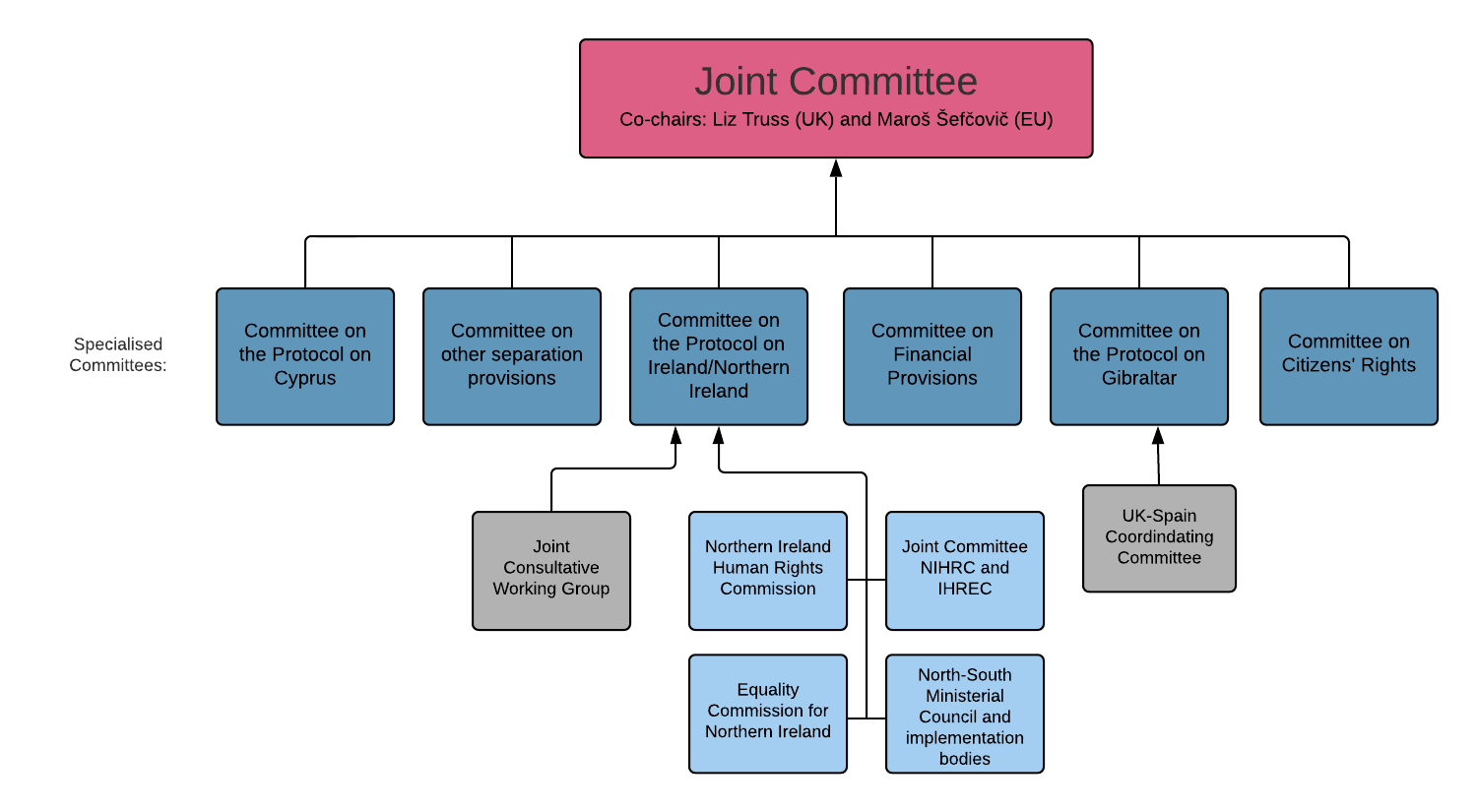
Governance structures of the EU-UK Withdrawal Agreement
SPS and customs
On SPS and customs proposals from the EU, Lord Frost stated these were also “a step forward but, based on what we have heard to date, our expert analysis does not support the ambitious public claims made for them.” Šefčovič said, “We are confident that our proposals [on SPS and customs] will deliver on their objectives, should the UK government reciprocate EU efforts and engage on both facilitating the movement of goods between Great Britain and Northern Ireland and on the safeguards needed to protect the EU Single Market.”
Talks between the EU and UK will resume in early January.
Committee hears evidence on medicines
On Thursday 16 December, the Committee for Health heard a similar sentiment to Lord Frost’s on medicines from officials from the Department of Health. NI’s Chief Pharmaceutical Officer Cathy Harrison outlined their concerns and those of industry in relation to medicines supply under the Protocol. She said the number of potential discontinuations of medicines had decreased as negotiations between the EU and UK got underway.
Harrison said the EU’s non-paper on medicines did not really provide all the detail needed to simply agree to the solution. She said the three areas of concern they have relate to new and innovative medicines, potential requirements for NI separate packaging – she highlighted that NI is a small market and there would be commercial decisions for companies - and what the EU single market protections would mean in practice. Harrison said it wasn’t good enough to have words said such as ‘creative solutions’: she needs to see the details and consider the implications for NI’s health service. The European Commission has since said that separate packaging will not be required and that NI will access novel medicines at the same time as the rest of the UK.
Regarding the UK’s position that medicines should be removed from the Protocol, Harrison said such a solution would make a lot of issues disappear, but emphasised that an agreed position is better long-term.
The Committee also heard evidence on Common Frameworks (see below).
Article 2
The Committee for the Executive Office on 15 December heard evidence on Article 2 of the Protocol on Ireland/Northern Ireland, which states that there shall be “no diminution of rights, safeguards or equality of opportunity” in Northern Ireland as a result of the UK’s exit from the EU. Alyson Kilpatrick, Chief Commissioner at the Northern Ireland Human Rights Commission, outlined their work on the scope of Article 2, especially regarding access to healthcare and all-island services.
Geraldine McGahey, Chief Commissioner at the Equality Commission for Northern Ireland said they have made progress in mapping EU law which may fall within the scope of Article 2. They are working on tracking and monitoring EU law and GB/NI law. The volume of enquiries received in relation to Article 2 is currently low, which is to be expected: more enquiries are likely as there is more divergence. Key concerns include access to halal meat, voting rights, and regulations affecting assistance dogs. The Commission is also examining Parliament and Assembly mechanisms to ensure effective legislative scrutiny in relation to Article 2. McGahey said it was necessary for Explanatory Memoranda for new legislative measures to make clear what consideration has been given to Article 2. On tracking changes to EU legislation, McGahey said they have been asking the EU to address this and noted the EU’s proposal for a website. She said it is a “mammoth task”.

Geraldine McGahey, Chief Commissioner at the Equality Commission for Northern Ireland | Source: NI Assembly
McGahey said they have been calling for a formal engagement process for civic society in relation to the Protocol’s governance structures. She said it is essential that the EU and UK Government continue to engage with civil society regarding Article 2, calling for more transparency in the Joint Consultative Working Group (JCWG). Kilpatrick said engagement needs to be structured and transparent and should focus on practical issues.
Funding of the Commissions was also discussed: Kilpatrick highlighted that the type and scope of work which they can carry out effectively is currently limited.
The House of Lords Sub-Committee on the Protocol last week wrote a lengthy letter to Northern Ireland Office Minister Conor Burns, asking for clarity on how the Article 2 commitments interact with the devolution settlement. The Committee also asks how resourcing for the Commissions will be sustained over time. It seeks further information from the Minister on greater engagement with the public regarding their Article 2 rights, the work of the JCWG and bodies which feed into the Protocol governance structures, scrutiny processes, divergence in legal rights, and UKG’s assessment of the interaction between the proposed changes to the Human Rights Act, Article 2, and the Belfast/Good Friday Agreement.
Common Frameworks for Health Policy
At the Committee for Health on Thursday, officials from the Department for Health also gave evidence on Common Frameworks. Frameworks are being developed in some areas where powers have returned from the EU and which intersect with devolved competence. The Public Health Protection and Health Security Framework provides a formal structure for collaboration between the four UK administrations on health security and health protection policy, including for collecting and sharing knowledge within the UK, and with the EU. Officials told the committee that this framework is, in effect, already running.
The Blood Safety and Quality Framework supports the maintenance of a compatible minimum set of high standards of safety and quality for blood and blood components across the UK. The Committee heard that while blood policy is devolved, NI must follow EU law and where the EU plans to make changes, NI will be obliged to follow. Officials highlighted that the EU is considering regulatory change in this areas at the moment and they will be bringing any changes into this framework for other devolved administrations to consider.
Regarding the Organs, Tissues and Cells Framework, the Committee heard that organ donation is managed on a UK-wide basis, and as organs move around the UK, it’s preferable to have a compatible system for tracing. If there is any divergence, they would have to use the framework to try and manage and agree on this.
UK-EU border
On 15 December, Lord Frost made a written statement on border controls. The UK Government is introducing full border controls with the EU over the course of 2022 but is extending temporarily the current arrangements for goods moving from the island of Ireland to GB. Frost stated, “Implementing these arrangements for goods moving from the island of Ireland, whether from Ireland or from Northern Ireland, is particularly complex. This is because there are specific Treaty and legislative commitments to “unfettered access” for goods from Northern Ireland, because there are currently “standstill” arrangements in place for operating the Northern Ireland Protocol, and because negotiations on the Protocol itself are still under way and will not be definitively completed by 1 January.” The Government believes “this pragmatic act of good will can help to maintain space for continued negotiations on the Protocol. It also ensures that traders in both Ireland and Northern Ireland are not faced with further uncertainty while the Protocol arrangements themselves are still under discussion.” The UK Government’s Border Operating Model has been updated to reflect this.
Taking questions from peers on 16 December, Lord Frost said, “In practice, at the moment, it is not always possible to distinguish between the two categories of goods [from NI or the Republic of Ireland], but that will change in future and we will need a definitive solution to this question. Of course, the degree of pragmatism that we show in future to Irish goods coming to Great Britain will be related to the degree of pragmatism and flexibility that the EU shows in allowing goods to move freely around all parts of the UK.”
Other news
- On Wednesday 15 December, a Westminster Hall Debate was held on ‘Securing a veterinary agreement in the Northern Ireland Protocol’. Minister for Farming, Fisheries and Food, Victoria Prentis said, “Dynamic alignment is not acceptable to this Government…We are in a position where our laws—not our standards, but our laws—have started to diverge from those of the EU.” Summing up the debate, Labour MP Tony Lloyd said, “the marginal changes that we have made in the short run were not worth causing so much damage to the economy of Great Britain and the economy of Northern Ireland…it is not just about Northern Ireland; it is also about the ability of Scottish, English and Welsh agrifood and agribusiness to export not simply to Northern Ireland but to the whole of the EU.”
- The UK Government has published its third quarterly report on Intergovernmental Relations. The report states that there has been “regular engagement between Ministers in Northern Ireland on matters relating to the Northern Ireland Protocol, addressing the past and implementing the New Decade, New Approach deal.”
- Professor Catherine Barnard has penned an article about the Review of Retained EU law (REUL) instigated by Lord Frost, explaining why it is important.
This Week at the Assembly
The Assembly is in recess until 9 January 2022.
Catch up with Assembly Business
- Monday 13 December, 2pm – Plenary - Question Time - The Executive Office
- Wednesday 15 December, 10am - Committee for Infrastructure - EU Exit legislation
- Wednesday 15 December, 2pm - Committee for The Executive Office - Article 2 of the Ireland/Northern Ireland Protocol: Dedicated Mechanism - Oral Briefing from NI Human Rights Commission, NI Equality Commission and Irish Human Rights and Equality Commission
- Thursday 16 December, 10.30am - Committee for Health - Briefing from the Department for Health on Common Frameworks and Brexit issues